What is Nitrosamine? (Nitrosamine)
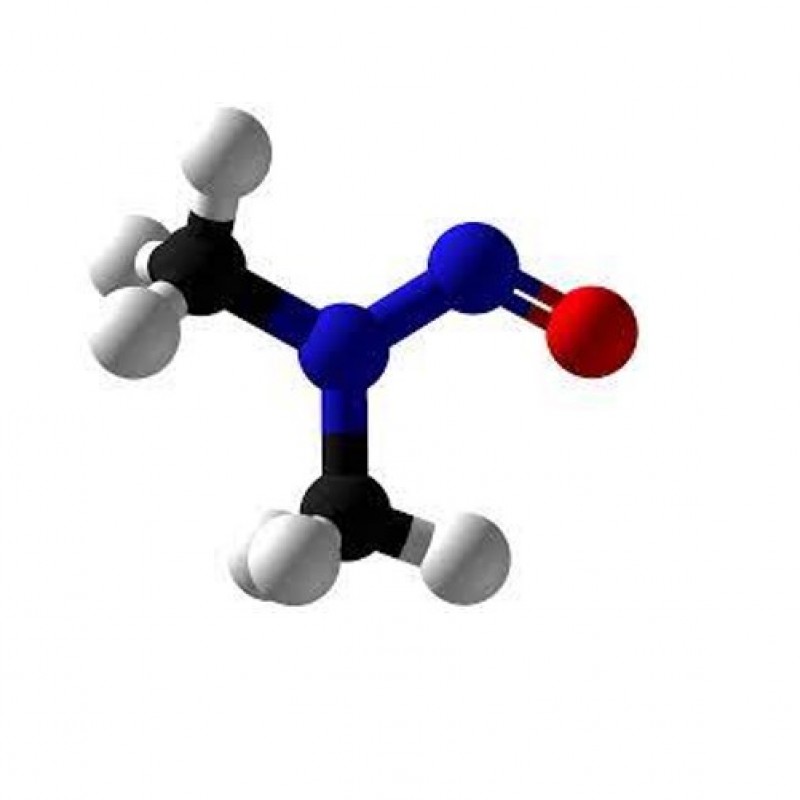
Nitrosamines (N-Nitrosamines) are organic compounds of the chemical structure R2N−N=O, where R is usually an alkyl group. They have a nitroso group (NO+) attached to an aprotic amine. Nitrosamines are carcinogens formed as a result of the in vivo reaction of nitrite preservatives with amines during the fermentation of various foods and tobacco and under acidic conditions in the gastrointestinal tract.
Organic chemistry of nitrosamines, they are generally produced by the reaction of nitrous acid (HNO2) and secondary amines.
HONO + R2NH → R2N-NO + H2O

Nitrous acid is usually caused by the protonation of a nitrite. This synthesis method is concerned with the production of nitrosamines under certain biological conditions.
The C2N2O core of nitrosamines is planar in structure, as determined by X-ray crystallography. N-N and N-O distances are 132 and 126 pm, respectively, in dimethylnitrosamine, one of the simplest members of a large class of N-nitrosamines.
Nitrosamines is a general term for a broad range of N-nitroso compounds (NOCs) that carry a common functional 4N-NQO group. NOCs are divided into N-nitrosamines and N-nitrosamides and related compounds.
N-Nitrosamines are N-nitroso derivatives of secondary amines (Fig. 1A); N-nitrosamides and related compounds are substituted ureas, amides, carbamates, guanidines and similar compounds (Fig. 1B).
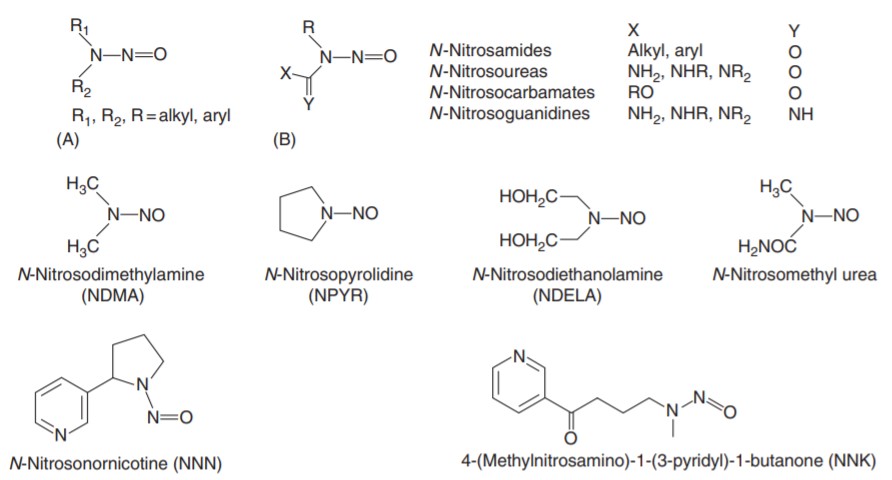
Şekil 1: Nitrozaminler ve nitrozamitler
What is NDMA? (N-Nitrosodimethylamine)
N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. NDMA (N-Nitrosodimethylamine), one of the simplest members of a large class of N-nitrosamines, is a volatile yellow oil. NDMA is highly hepatotoxic and carcinogenic.
NDMA and other nitrosamines are ubiquitous in small amounts in outdoor air, water, and soil. They are formed by the chemical interaction of a substituted (secondary or tertiary) amine with an oxidizing agent, usually a nitrite.
NDMA consists of various dimethylamine-containing compounds; hydrolysis of dimethylformamide. Dimethylamine is susceptible to oxidation to unsymmetrical dimethylhydrazine, which oxidizes to NDMA in air. In the laboratory, NDMA can be synthesized by the reaction of nitrous acid with dimethylamine:
HONO + (CH3)2NH → (CH3)2NNO + H2O
The carcinogenicity mechanism includes metabolic activation steps that result in the formation of methyl diazonium, an alkylating agent.
In foods, the nitrosating agent responsible for the formation of NDMA is nitrous anhydride, which usually originates from a nitrite in an acidic aqueous solution, such as in the stomach. Beer, processed meats like bacon or sausages, tobacco (with or without smoke), and even water contain small amounts of nitrosamines. Nitrosamines have been evaluated for carcinogenic activity and positive results have been obtained in many animal species as they induce tumors in the liver, kidney and respiratory tract.
The specific contaminant NDMA discovered in drugs has produced cancer in a number of experimental animal species and caused cirrhosis and hyperplastic nodules in monkeys. Based on this evidence, nitrosamines, including NDMA, have been classified as probable carcinogens in humans.
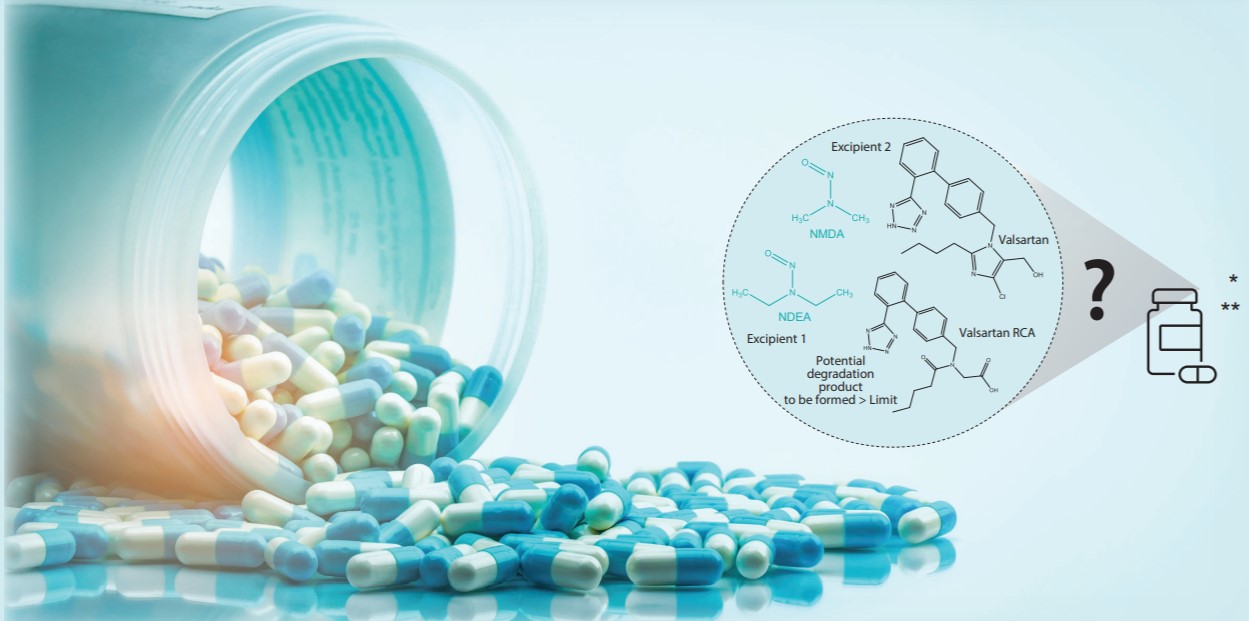
Due to raw materials such as sodium nitrite, dimethylformamide and triethylamine, the presence of nitrosamines as undesirable by-products of production in drugs has occurred. NDMA and NDEA are classified as Group 2A (possibly carcinogenic to humans) according to the International Agency for Research on Cancer (IARC) carcinogenicity classification. Applying the ICH M7 guideline concept to the management of carcinogenic impurities, NDMA and NDEA are classified as Class 1 in ICH M7 and must be managed at or below compound-specific allowable levels.