What is protein electrophoresis?
Protein electrophoresis is a standard laboratory technique in which charged protein molecules are transported through a solvent by an electric field. Both proteins and nucleic acids can be separated by electrophoresis, a simple, fast and sensitive analytical tool.
Most biological molecules carry a net charge at any pH other than their isoelectric point and migrate at a rate proportional to their charge densities. The mobility of a molecule through an electric field depends on factors such as field strength, net charge on the molecule, size and shape of the molecule, ionic strength, and properties of the matrix (eg, viscosity, pore size) through which the molecule passes.
Polyacrylamide and agarose are two support matrices commonly used in electrophoresis. These matrices act as porous media and act as molecular sieves. Agarose has a large pore size and is suitable for separating nucleic acids and large protein complexes. Polyacrylamide has a smaller pore size and is ideal for separating most proteins and smaller nucleic acids.
What are polyacrylamide gels?
Polyacrylamide is the material of choice for preparing electrophoretic gels to separate proteins by size. Polyacrylamide gels are prepared by mixing acrylamide with bisacrylamide to form a cross-linked polymer network when the polymerizing agent, ammonium persulfate (APS), is added. TEMED (N,N,N',N'-tetramethylenediamine) catalyzes the polymerization reaction by promoting the production of free radicals by APS. At this stage it becomes polyacrylamide.

Native-PAGE with SDS-PAGE (denaturing)
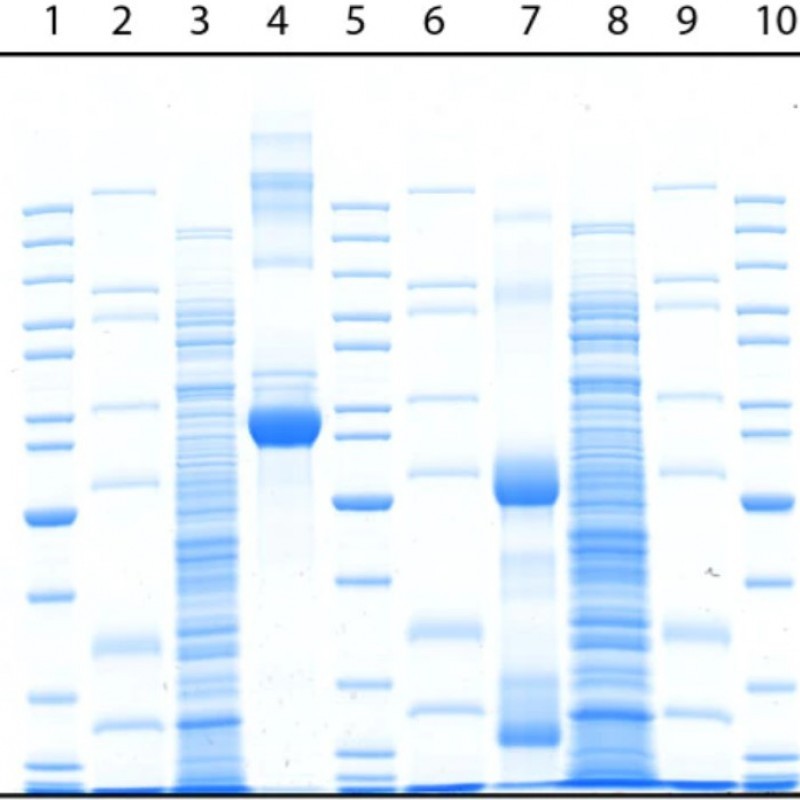
SDS-PAGE
In SDS-PAGE, the gel is poured into a buffer containing sodium dodecyl sulfate (SDS), an anionic detergent. SDS denatures proteins by wrapping the polypeptide backbone. By heating the protein sample to 70-100°C in the presence of excess SDS and thiol reagent, the disulfide bonds are cleaved and the protein completely dissociates into its subunits. Under these conditions, most polypeptides bind SDS at a fixed weight ratio (1.4 g SDS: 1 g polypeptide). The intrinsic charges of the polypeptide are negligible compared to the negative charges provided by the bound detergent, so that the SDS-polypeptide complexes have essentially the same negative charge and shape. As a result, proteins pass through the gel strictly by polypeptide size, with little effect from compositional differences. The simplicity and speed of this method, as well as the fact that only microgram amounts of protein are required, have made SDS-PAGE the most widely used method for determining the molecular mass in a polypeptide sample. Proteins from almost any source are readily solubilized by SDS, so the method is generally applicable.
When a set of proteins of known mass are run alongside samples in the same gel, they provide a reference from which the mass of the sample proteins can be determined. These reference protein sets are called mass markers or molecular weight markers (MW markers), protein ladders, or size standards, and they are commercially available in a variety of forms.
Native-PAGE
In Native-PAGE, proteins are separated according to the net charge, size, and shape of their native structure. Electrophoretic migration occurs because most proteins carry a net negative charge in alkaline working buffers. The higher the negative charge density (more charge per molecular mass), the faster a protein will migrate. At the same time, the friction force of the gel matrix creates a sieving effect by regulating the movement of the proteins according to their size and three-dimensional shape. Small proteins face only a small frictional force, while larger proteins face a greater frictional force. Thus, native-PAGE separates proteins according to both their charge and mass.
Since no denaturants are used in Native-PAGE, subunit interactions within a multimeric protein are usually preserved and information about the quaternary structure can be obtained. In addition, some proteins retain their enzymatic activity (function) after separation by Native-PAGE. Thus, this technique can be used for the preparation of purified, active proteins.

Following electrophoresis, proteins can be recovered from a native gel by passive diffusion or electroelution. To preserve the integrity of the proteins during electrophoresis, it is important to keep the apparatus cool and to minimize denaturation and proteolysis. Extreme pH values should generally be avoided in native-PAGE because they can cause irreversible damage to the proteins involved, such as denaturation or aggregation.
Protein Ladders and Standards
To evaluate the molecular mass (sizes) of proteins in a gel, a prepared mixture containing several proteins with known molecular masses is run along one or more strips of the gel with the test sample. Such known protein aggregates are called protein molecular weight (or mass) markers or protein ladders. A standard curve can be constructed from the distances carried by each marker protein. The distance traveled by the unknown protein is then plotted and the molecular weight estimated from the standard curve.
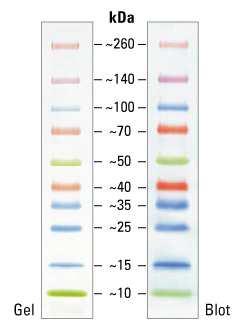
Several types of off-the-shelf protein molecular weight (MW) markers are available, either unlabeled or pre-stained for different detection modes. They are pre-reduced and therefore primarily suitable for SDS-PAGE rather than native PAGE. MW markers can also be made detectable by special tags such as fluorescent labels and other methods.